| CAS
NO. |
3524-62-7 |
|
| EINECS
NO. |
222-538-7 |
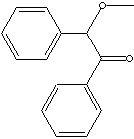
| FORMULA |
C6H5CHOCH3COC6H5 |
| MOL
WT. |
226.27 |
| H.S.
CODE |
2914.50 |
| SMILES |
|
| TOXICITY |
|
| SYNONYMS |
2-Methoxy-2-phenylacetophenone;
o-Mthylbenzoin; |
| alpha-Methoxydeoxybenzoin;
Benzoin Methyl ether; Methoxy phenylacetophenone; 2-Methoxy-1,2-diphenylethanone;
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
to off-white powder |
| MELTING
POINT |
47
- 49 C |
| BOILING
POINT |
188
- 189 C at hPa 15 |
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| AUTOIGNITION
|
|
| pH |
|
| VAPOR
DENSITY |
|
| NFPA
RATINGS |
Health:
1; Flammability: 0; Reactivity: 0 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
>
110 C |
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
Polymerization is initiated generally by free radical. There are four types of
free radical initiators which initiate polymerization.
- Peroxides including
hydroperoxides (tertiary-butyl hydroperoxide, benzoylperoxide)
- Azocompound
thermal initiators (azoisobutyronitrile)
- Redoxinitiators (mixture of
iron(III) acetylacetonate): free radicals are formed by one-electron
transferreactions. Useful in low temperature and emulsion
polymerization
- Photoinitiators (benzoin, benzil dimethylketal)
The main
advantage of polymerization started by photoinitiators is
temperature-independence and easy control. It can be conducted at very low
temperatures and can be stopped simply by removing the light source.
Photoinitiators are compounds that break down into free radicals upon exposure
to ultraviolet radiation. Photoinitiators undergo a unimolecular bond cleavage
upon irradiation to yield free radicals (benzoin esters; benzil ketals;
alpha-dialkoxy acetophenones; alpha-hydroxy-alkylphenones; alpha-amino alkyl-
phosphine; acylphosphine oxides). Another type of photoinitiators undergo a
bimolecular reaction where the excited state of the photoinitiator interacts
with a second molecule (a coinitiator) to generate free radicals(benzo
phenones,amines; thioxanthones,amines; titanocenes). Photoinitiators are widely
applied in UV curing inks, wood coatings, paper coatings, optical fiber, PCB,
screen printing , paper varnish and other surface coatings.
Benzoin is a highly toxic crystalline substance with formula of
C6H5CH(OH)COC6H5,
alpha-hydroxy aromatic ketone. It is a white to yellowish
crystals; slightly soluble in water, soluble in acetone; melting at 137 C. It
is prepared by the condensation of benzaldehyde in an alkaline cyanide
solution. Benzoin and its derivatives are used as intermedaites for the
synthesis of organic compounds and as catalysts in photopolymerization. They
are used for anticratering in powder coatings. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white
to off-white powder |
|
ASSAY
|
98.0%
min
|
| MELTING
POINT |
56
- 59 C |
| TRANSPORTATION |
| PACKING |
50kgs
in Fiber drum |
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
| European
Hazard Symbols: XN, Risk Phrases: 22, Safety Phrases:
22 |
|
GENERAL
DESCRIPTION OF ETHER
|
| Ether is any of a number of organic compounds characterized by an oxygen atom
joined with single bonds by two carbon atoms that are part of hydrocarbon
groups. The general formula is R-O-R', where R and R' are alkyl or aromatic
groups. Ethers are formed by the condensation of two alcohols by heating with
sulfuric acid; the reaction is one of dehydration. Ethers can be prepared from
alkyl halide reacted with metallic alkoxide (called Williamson synthesis).
Ethers are similar to alcohols but are generally less dense, less soluble in
water, and have lower boiling points. They are relatively unreactive, which
makes them valuable solvents. But ethers will be
cleaved at high temperatures by concentrated hydrogen halides. Ethers have relatively low boiling point
compare to alkanes as they don't form hydrogen bonds each other. Ethers are more
lipophilic than esters [R-C(=O)-O-R']or amides [RCO-NH2]. Ethers are widely used as solvents for various organic reactions because they
are relatively the least reactive among common organic compounds except alkanes
and fluorocarbons. The common reaction of ethers is cleavage of the C–O bond by
strong acids either in linear chain or cyclic structure. Ethers in which oxygen
is bonded to primary and secondary alkyl groups can form peroxide compounds in
the presence of gaseous oxygen due to two unpaired electrons in oxygen. Ethers can act as
Lewis bases in chemical reactions. Commonly, ethers are
named simply in listing the alkyl groups in alphabetical order or alkane order
such as ethyl methyl ether or methyl ethyl ether, which is methoxyethane in
IUPAC nomenclature ( the formula of "alkoxyalkane" ). When ether is a
parts of complex molecule or aromatic derivatives, it is described as an alkoxy
substituent such as methoxybenzene ( trivial name is anisole). The methoxy
prefix indicates the function methyl group joined by single bonds to an oxygen atom,
with the general formula -O-CH3. Cyclic ethers
have ring structure where the oxygen has become part of the ring. The term of
epoxide indicate three membered cyclic ether (also called oxirane) in which an
oxygen atom is joined to each of two carbon atoms that are already bonded to
each other; four
membered cyclic ether is called oxetane; five membered cyclic ether, furan (or
oxolane); six membered cyclic ether, pyran (also called oxane) respectively.
Their unhindered oxygen atom carries two unshared pairs of electrons - a
structure which favors the formation of coordination complexes and the solvation
of cations. Cyclic ethers are used as important solvents, as chemical
intermediate and as monomer for ring-opening polymerization. Crown Ether is a macrocyclic polyether whose structure contains
hydrogen, carbon and oxygen atoms. Each oxygen atoms are confined between two
carbon atoms and exhibits a conformation with a hole (accordingly called
"crown"). Anisole is one of the simplest aromatic compound to
which ether group is linked. But
it is different with aromatic compounds like furan where the oxygen is a part of
the ring. Anisole, C6H5OCH3
(methyl phenyl ether), is a clear liquid that is soluble in ether and
alcohol; insoluble in water; boiling point 155 C. Anisole and its derivatives
are used as solvents and in perfumery. Anisole can be obtained from anise seed.
Anisic acid, p-methoxybenzoic acid, is a part of cresol class antiseptic
compounds. It
is also used as an insect repellent and ovicide.
Anisole, anisic acid, and their derivatives are also widely used in
chemical reaction as intermediates to obtain target materials such as dyes,
pharmaceuticals, perfumes, photoinitiators and agrochemicals. |
